The compressibility factor of air is a measure of how much it deviates from behaving like an ideal gas. It helps us understand why air doesn't always follow simple gas laws. The compressibility factor is a number that tells us if air is more or less compressible than expected.
Table of content:
Compressibility factor
Compressibility factor of air
Compressibility factors for air at different temperature and pressure conditions
Compressibility factor
The compressibility factor, often denoted as Z, is a dimensionless quantity that describes the deviation of a real gas from the ideal gas behavior. It is defined as the ratio of the actual molar volume of a gas to the molar volume predicted by the ideal gas law at the same temperature and pressure. Mathematically, it can be expressed as:
Z = (PV) / (RT)
Where: Z is the compressibility factor, P is the pressure of the gas, V is the volume occupied by the gas, R is the gas constant, and T is the temperature of the gas.
Why is the Z factor important?
For an ideal gas, the compressibility factor is always equal to 1. However, for real gases, the compressibility factor can deviate from 1 due to intermolecular forces and the finite volume occupied by gas molecules.
The compressibility factor can provide important information about the behavior of real gases. For example, if Z is greater than 1, it indicates that the gas is more compressible than predicted by the ideal gas law. Conversely, if Z is less than 1, the gas is less compressible than expected.
The compressibility factor is often used in thermodynamics and fluid dynamics to correct for non-ideal behavior when calculating gas properties such as volume, pressure, and temperature. Experimental data and equations of state are used to determine or estimate the compressibility factor for specific gases. under different conditions.
Compressibility factor of air
The compressibility factor of air depends on the specific conditions of pressure and temperature. However, at moderate pressures and temperatures, air can be approximated as an ideal gas, which means its compressibility factor is close to 1.
Under normal conditions of around 1 atmosphere (101.325 kPa) and room temperature (around 25 degrees Celsius or 298 Kelvin), the compressibility factor of air is very close to 1. This approximation holds well for many practical engineering and scientific calculations.
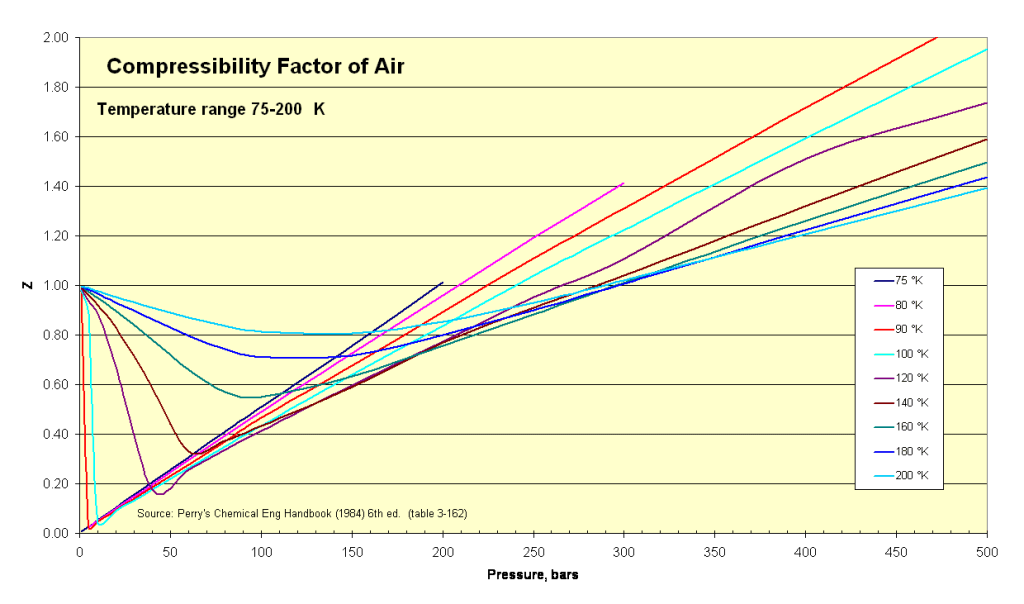
However, at extreme conditions of high pressures or low temperatures, the compressibility factor of air deviates from 1 due to the effects of intermolecular forces and molecular interactions. In such cases, the compressibility factor can be determined using more complex equations of state or experimental data specific to those conditions.
Compressibility factors for air at different temperature and pressure conditions
Table1: 1bar - 80bar
| Pressure, bar (absolute) | |||||||
| Temp, K | 1 | 5 | 10 | 20 | 40 | 60 | 80 |
| 75 | 0.0052 | 0.0260 | 0.0519 | 0.1036 | 0.2063 | 0.3082 | 0.4094 |
| 80 | 0.0250 | 0.0499 | 0.0995 | 0.1981 | 0.2958 | 0.3927 | |
| 90 | 0.9764 | 0.0236 | 0.0453 | 0.0940 | 0.1866 | 0.2781 | 0.3686 |
| 100 | 0.9797 | 0.8872 | 0.0453 | 0.0900 | 0.1782 | 0.2635 | 0.3498 |
| 120 | 0.9880 | 0.9373 | 0.8860 | 0.6730 | 0.1778 | 0.2557 | 0.3371 |
| 140 | 0.9927 | 0.9614 | 0.9205 | 0.8297 | 0.5856 | 0.3313 | 0.3737 |
| 160 | 0.9951 | 0.9748 | 0.9489 | 0.8954 | 0.7803 | 0.6603 | 0.5696 |
| 180 | 0.9967 | 0.9832 | 0.9660 | 0.9314 | 0.8625 | 0.7977 | 0.7432 |
| 200 | 0.9978 | 0.9886 | 0.9767 | 0.9539 | 0.9100 | 0.8701 | 0.8374 |
| 250 | 0.9992 | 0.9957 | 0.9911 | 0.9822 | 0.9671 | 0.9549 | 0.9463 |
| 300 | 0.9999 | 0.9987 | 0.9974 | 0.9950 | 0.9917 | 0.9901 | 0.9903 |
| 350 | 1.0000 | 1.0002 | 1.0004 | 1.0014 | 1.0038 | 1.0075 | 1.0121 |
| 400 | 1.0002 | 1.0012 | 1.0025 | 1.0046 | 1.0100 | 1.0159 | 1.0229 |
| 450 | 1.0003 | 1.0016 | 1.0034 | 1.0063 | 1.0133 | 1.0210 | 1.0287 |
| 500 | 1.0003 | 1.0020 | 1.0034 | 1.0074 | 1.0151 | 1.0234 | 1.0323 |
| 600 | 1.0004 | 1.0022 | 1.0039 | 1.0081 | 1.0164 | 1.0253 | 1.0340 |
| 800 | 1.0004 | 1.0020 | 1.0038 | 1.0077 | 1.0157 | 1.0240 | 1.0321 |
| 1000 | 1.0004 | 1.0018 | 1.0037 | 1.0068 | 1.0142 | 1.0215 | 1.0290 |
Table2 - 100bar - 500bar
| Pressure, bar (absolute) | ||||||||||||||
| Temp, K | 100 | 150 | 200 | 250 | 300 | 400 | 500 | |||||||
| 75 | 0.5099 | 0.7581 | 1.0125 | |||||||||||
| 80 | 0.4887 | 0.7258 | 0.9588 | 1.1931 | 1.4139 | |||||||||
| 90 | 0.4681 | 0.6779 | 0.8929 | 1.1098 | 1.3110 | 1.7161 | 2.1105 | |||||||
| 100 | 0.4337 | 0.6386 | 0.8377 | 1.0395 | 1.2227 | 1.5937 | 1.9536 | |||||||
| 120 | 0.4132 | 0.5964 | 0.7720 | 0.9530 | 1.1076 | 1.5091 | 1.7366 | |||||||
| 140 | 0.4340 | 0.5909 | 0.7699 | 0.9114 | 1.0393 | 1.3202 | 1.5903 | |||||||
| 160 | 0.5489 | 0.6340 | 0.7564 | 0.8840 | 1.0105 | 1.2585 | 1.4970 | |||||||
| 180 | 0.7084 | 0.7180 | 0.7986 | 0.9000 | 1.0068 | 1.2232 | 1.4361 | |||||||
| 200 | 0.8142 | 0.8061 | 0.8549 | 0.9311 | 1.0185 | 1.2054 | 1.3944 | |||||||
| 250 | 0.9411 | 0.9450 | 0.9713 | 1.0152 | 1.0702 | 1.1990 | 1.3392 | |||||||
| 300 | 0.9930 | 1.0074 | 1.0326 | 1.0669 | 1.1089 | 1.2073 | 1.3163 | |||||||
| 350 | 1.0183 | 1.0377 | 1.0635 | 1.0947 | 1.1303 | 1.2116 | 1.3015 | |||||||
| 400 | 1.0312 | 1.0533 | 1.0795 | 1.1087 | 1.1411 | 1.2117 | 1.2890 | |||||||
| 450 | 1.0374 | 1.0614 | 1.0913 | 1.1183 | 1.1463 | 1.2090 | 1.2778 | |||||||
| 500 | 1.0410 | 1.0650 | 1.0913 | 1.1183 | 1.1463 | 1.2051 | 1.2667 | |||||||
| 600 | 1.0434 | 1.0678 | 1.0920 | 1.1172 | 1.1427 | 1.1947 | 1.2475 | |||||||
| 800 | 1.0408 | 1.0621 | 1.0844 | 1.1061 | 1.1283 | 1.1720 | 1.2150 | |||||||
| 1000 | 1.0365 | 1.0556 | 1.0744 | 1.0948 | 1.1131 | 1.1515 | 1.1889 | |||||||
FAQs
1. How does the compressibility factor change with varying conditions?
The compressibility factor for air, like most gases, varies with changes in pressure and temperature. As pressure and temperature increase, gases tend to deviate more from ideal behavior. When approaching extreme conditions (very high pressure or very low temperature), the compressibility factor can significantly deviate from 1.
2. What is the significance of compressibility factor in the oil and gas industry?
In the oil and gas industry, understanding the compressibility factor is crucial for reservoir engineering, production, and transportation of natural gas. It helps determine the volume of gas at different pressures and temperatures, allowing for accurate volume calculations, which is vital for resource assessment and pipeline design.
3. Can the compressibility factor be measured experimentally?
Yes, compressibility factor can be measured experimentally using specialized equipment such as PVT (pressure-volume-temperature) cells. These measurements provide valuable data for the development of accurate equations of state for gases under various conditions.
4. Where can I find compressibility factor charts and tables for air?
You can find compressibility factor charts and tables in thermodynamic reference books, engineering handbooks, or online resources. These references help engineers and scientists quickly estimate Z for air under different conditions.
Understanding the compressibility factor for air is essential in various fields, from chemical engineering to meteorology. It allows for precise calculations and predictions of gas behavior under different conditions, ensuring safe and efficient processes in numerous industries.