Catalytic gas detectors are probably the ones with the most widespread use nowadays. Their main advantages over other methods of combustible gas detection are simplicity, accuracy and relatively low-cost.
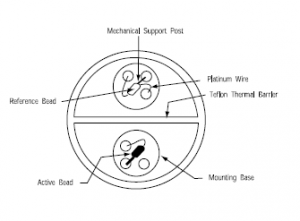
Catalytic elements (beads) typically consist of a platinum coil inserted in a catalyst.
Their operation is quite straight-forward: they work on the principle that a combustible gas can be exothermically oxidized: one sensor of this type typically consists of two identical beads, one active, that oxidises the combustible gas present and the second, the reference bead, which is used for reference and that is typically glass-coated. Glass coating allows it to respond to temperature and pressure changes but renders it immune to the presence of combustible gases (combustible gases can not penetrate the glass coating). As gas oxidizes on the active bead, its temperature increases and ,when compared to the reference bead resistance of the reference bead, results to a measurable voltage difference. This voltage difference is then interpreted to a signal, which is then used in order to initiate alarms and/or adequate fire prevention measures.
Sensors could be damaged as a result of shock or vibration, leading to breaking of the platinum wire. In order to protect any type of mechanical damage and extend sensor life, the beads of high quality catalytic sensors are mounted on a support
Picture 1 - Typical catalytic detector layout
The catalyst emplosed is very critical for the accuracy and prolonged lifetime of the sensor. The sensitivity of catalytic gas detectors is mainly affected by the contamination or poisoning of the active bead.
Contamination of the sensor is mostly dependend on the environment in which the sensor is installed and may be caused by a variety of reasons like for example exposure to dust, particulate matter, salty/marine environment, heavy oi, grease, paint or varnish vapors. Poisoning of the catalytic element actually ''neutralises'' the active bead. Various substances can act as catalyst poisons, like for example silicon and sulfur compounds, stong acids, bases and heavy metals. The only way of indentifying catalytic poisoning is by proper gas-checking and calibration. A recommended calibration interval is typically provided by the manufacturer and shall be closely adhered by in the field.