Requirement of Carbon capture and Sequestration
The amount of CO2 in the environment is increasing day by day due to human activity. This is causing earth to warm and ocean to become more acidic. Unless the amount of CO2 and other global warming agents are not reduces in the environment, scientists predict that temperature of earth will keep on increasing.
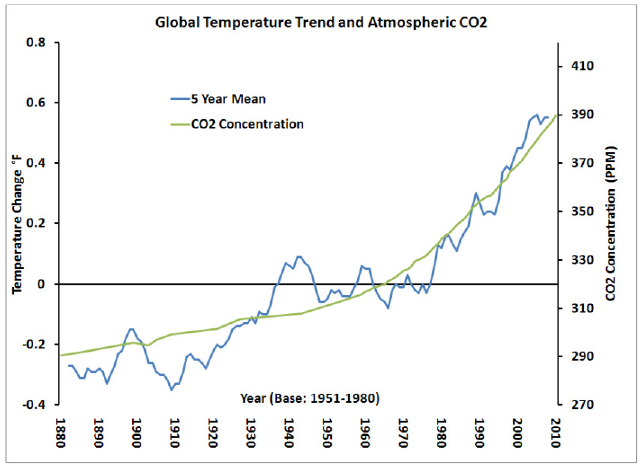
Source: http://www.c2es.org/facts-figures/trends/co2-temp
From this graph, it is evident that temperature increase in the environment is directly related with CO2 concentration in the environment. The rise in temperature will cause sea level to rise, melting snow in different glaciers and this will adversely affect land mass earth and endanger human lives.
Natural role of CO2
CO2 is essential for the existence of mankind on earth. It is part of food supply chain and plants uses it in the photo-synthesis process. A critical level of CO2 is required to make sure that all sun-light doesn’t escape to space and this sun-light keeps earth warm and inhabitable for different species to survive.
Carbon dioxide (CO2) is the main culprit for the greenhouse effect. A finite amount of carbon is stored in fossil fuels, the sea, living matter and the atmosphere. Without human influence, transfers between these stores roughly balance each other – for example, plants absorb carbon as they grow, but release it as they decay. But when humans cut down trees or burn fossil fuels, they release extra carbon into the atmosphere, increasing the greenhouse effect. This is called carbon-cycle.
Non traditional sources of CO2
When fossil fuel burns, in order to generate electricity, a high concentration of CO2 gas is released into the environment. CO2 is also produced along with petroleum fluids, along with the production of crude oil and natural gas. Industrial process, such as, refining iron, melting steel and different type of industries also release a big chunk of CO2 in the environment. Other major source of CO2 is from the daily transportation activity, when petroleum products are burned.
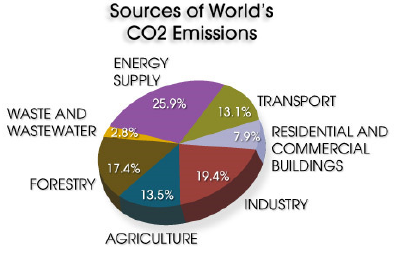
Source: http://www.pbs.org/wgbh/pages/frontline/heat/etc/worldco2.html
So, for survival of human race we need to make changes in the way we generate electricity, do transportation and heat our homes. One way of change is to develop renewable energy sources, switching to less carbon-intensive and more energy efficient fuels. Since, no renewable energy source is commercially available on broad scale and fossil fuels are still expected to be used for at least next 100 years to generate electricity; it is advisable to do something to reduce emissions generated from power plant and different industries.
Carbon capture and Storage
Carbon capture and storage (CCS), refers to technologies attempting to prevent the release of large quantities of CO2 into the atmosphere. CO2 generated from fossil fuel burning in power generation and other industries can be captured, transported and ultimately, pumped into underground geological formations to securely store it away from the atmosphere. This is one way to mitigate rising global warming by capturing CO2 and storing it. Most of the technologies needed for CCS are already being used extensively in a variety of industries, but are yet to be widely applied to power generation and industry at commercial scale.
Capturing CO2 is most effective at point sources such as the emitting point of large fossil fuel based plant, industries with major CO2 emissions and in the production of H2 generation. Extraction of CO2 from air is possible but not very economical. There are several methods to capture CO2 but all of those haven’t been optimized for commercial applications. In the past focus has been on the capture of pure CO2 for other industrial usage rather than reducing CO2 concentration in the flue gas coming out of industries.
Carbon capture technologies
Post combustion capture
In post combustion capture technology, CO2 is captured after combustion of fossil fuel. Here carbon-dioxide is captured from flue gases from the emitting point of large industries. This is well understood technology and several demonstration plants are operational ;though not at the commercial scale.
Pre-combustion capture
This technology is widely applied in fertilizer, chemical and H2 production. In these cases, fuel is partially oxidized and it produces syn-gas. Syn-gas is a mixture of CO, CO2 and H2. Through water gas shift reaction, CO2 is again produced which is captured using different methods and H2 is produced. Through this method, we get relatively pure CO2. In this method, capture of CO2 takes place before the combustion process.
Oxy-fuel combustion
In this process, fuel is burned using oxygen rather than air. To control the temperature level inside the combustion chamber cool flue gas is sent. Because of the burning of fuel in presence of oxygen, flue gas consists only carbon di-oxide and water vapour. Water vapour is cooled so only pure carbon dioxide is left, which is sent for storage and then for sequestration or industrial usage.
Chemical looping combustion
In this method of CO2 sequestration rather than oxygen, metal oxide acts as carrier of oxygen in the combustion chamber. The flue gas consists mostly of pure CO2 and water vapour. Water vapour is then cooled leaving pure CO2. The pure CO2 is sent for sequestration or industrial usage. Solid metal particles are sent to reactor where they react with air, which oxidises the metal particle and they are ready to be reused again.
Transport
After capturing, CO2 is transported to sequestration sites through pipeline which is the most cheapest way.
CO2 storage
Various forms have been conceived for permanent CO2 storage. This includes gaseous storage in various deep geological sites, liquid form storage in deep ocean and solid storage of CO2 with reaction to metal oxides and formation of stable carbonates.
Geological storage
In this method, CO2 in form of supercritical condition is injected into underground geological formations. Oil fields, gas fields, saline formations, unmineable coal seams, and saline-filled basalt formation has been suggested as possible storage sites.
CO2 storage in ocean
Several different methods have been proposed to store CO2 inside ocean.
• In the method ‘dissolution’ CO2 is injected into ocean at depths of 1000-3000 mtr. By ship or pipeline, and subsequently CO2 dissolves in seawater.
• In another method CO2 is injected at depths greater than 3000 mtr. Inside ocean. Because of the high pressure at such depths, CO2 liquifies and forms a kind of lake over there. This process inhibits dissolution of CO2 into the atmosphere and sea for over a millennium years.
• In another method CO2 is made to react with carbonate salt to make bicarbonate and this is deposited in ocean.
• Store the CO2 in solid clathrate hydrates in the ocean.
Mineral Storage: in this process, CO2 is exothermally reacted with metal oxides to form stable carbonates. This process takes years of time and is responsible for large deposits of limestone on earth. With the help of high pressure and temperature, this process can be expedited.





