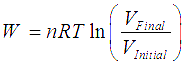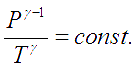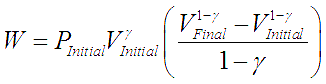A system can reach from state A to state B following different paths. These paths followed by a system while undergoing a change of state, are termed as thermodynamic processes. Some peculiar classes of thermodynamic processes are characterized by holding some of the thermodynamic variables constant through the path of the process. Thermodynamic work done to get from state A to B depends on the path taken.
Isobaric Process (Constant Pressure)
Thermodynamic work done in an isobaric process is given by,
For a closed system with ideal gases the work becomes,
Isochoric Process (Constant Volume)
Thermodynamic work done in an isochoric process is given by,
![]() , since volume is held constant.
, since volume is held constant.
Thus for an isochoric process the work done is always zero. A typical example of an isochoric process is addition or removal of heat from a closed system. The volume remains constant but temperature and pressure change according to the process.
Isothermal Process (Constant Temperature)
Work done in an isothermal process is given by,
Thus for and expanding system work done is negative and for a shrinking system external work done is positive.
Adiabatic Process (No Heat Transfer)
An adiabatic system is perfectly insulated from external environment and there is no heat transfer in or out of the system. Work done in an adiabatic process completely results in change in the internal energy of the system.
A special case in adiabatic processes is a ‘Reversible adiabatic process’. The following equation describes such a system for an ideal gas,
where ![]() is the adiabatic index or specific heat ratio of the gas.
is the adiabatic index or specific heat ratio of the gas.
For a reversible adiabatic process, the system entropy remains unchanged. This type of processes is also known as ‘Isentropic processes’.
Another special case of adiabatic processes is ‘Isenthalpic processes’. An isenthalpic process is an adiabatic irreversible process which extracts no external work.
Isentropic Process (Constant Entropy)
Isentropic process is characterized by constant entropy of the system. It is a special case of the adiabatic processes, defined as a reversible adiabatic process. For an ideal gas undergoing isentropic process,
or
or
Thermodynamic work done on a system undergoing isentropic process is given by,
where ![]() is the adiabatic index or specific heat ratio of the gas.
is the adiabatic index or specific heat ratio of the gas.
Isenthalpic Process (Constant Enthalpy)
Isenthalpic process is characterized by constant enthalpy of the system. It is a special case of the irreversible adiabatic processes, in which no external work is extracted. Thus work done in an isenthalpic process is zero.