Galvanic corrosion can occur in piping and equipment systems when two dissimilar metals are joined together. Galvanic corrosion occurs due to an electrochemical process in which one metal corrodes preferentially to another.
For galvanic corrosion to occur, two dissimilar metals need to be in contact with each other and with an electrolytic fluid. When two dissimilar metals are in contact with the same electrolyte, they act as electrodes with different different potential.
In such an arrangement, one metal acts as cathode and the other acts as an anode, the electrolytic fluid provides a medium for ion exchange between the two betals based on the electrode potential difference.
Galvanic Series
Different metals are arranged in a galvanic series representing the electrode potential for a given metal-electrolyte combination against the potential of a standard reference electrode. The relative positions of two different metals on such a galvanic series predicts the possibility and extent of galvanic corrosion when those two metals are connected.
Insulating Gaskets
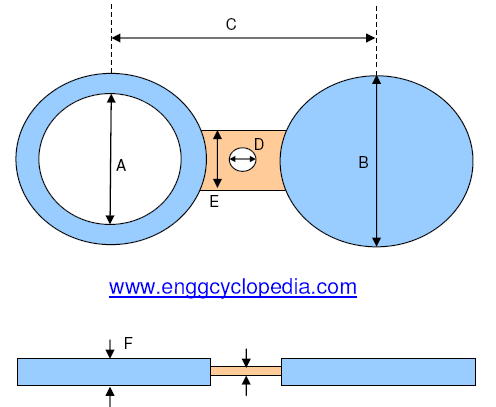
Two prevent galvanic corrosion from occurring in a piping systems, insulating gaskets can be placed between flanges of two different metals. Care has to be taken not to accidentally remove these insulating gaskets when they are placed right next to piping accessories (valves, blinds etc.), which are moved from their position frequently.
Sacrificial Anode
Another way to prevent galvanic corrosion is to use a Sacrificial Anode. Sacrificial anode is made of another different metal which is more active on the galvanic series, compared to the protected metal. The more active metal of sacrificial anode gets preferentially corroded instead of the protected metal and has to be regularly replaced. Sacrficial anodes are commonly places in equipments such as, tanks, heaters etc.
Other ways to prevent galvanic corrosion
Galvanic corrosion can also be prevented from occurring by, keeping the piping system dry and shielded from electrolytes such as salts, acids etc. This can be achieved by painting or coating either one or both the metals in question.
Another way to prevent galvanic corrosion is not to join two different metals together which are far apart from each other in the galvanic series.