Refrigerants are the coolant gases used in refrigeration process. They undergo compression, followed by expansion across a JT Valve. This expansion cools them down substantially, so that they can be used to lower the temperature of other fluids, commonly air.
Let's look at different types of refrigerants and when certain types are considered applicable.
Types of refrigerants
The most common types of refrigerants in use nowadays are listed below:
- Halocarbons or freons.
- Azeotropic refrigerants
- Zeotropic refrigerants
- Inorganic refrigerants like carbon dioxide, ammonia, water and air
- Hydrocarbon refrigerants.
Halocarbons are generally synthetically produced. Depending on whether they include chemical elements hydrogen (H), carbon (C), chlorine (Cl) and florine (F) they are named after as follows:
CFCs (Chlorofluorocarbons): R11, R12, R113, R114, R115
HCFCs (Hydrochlorofluorocarbons): R22, R123
HFCs (Hydrofluorocarbons): R134a, R404a, R407C, R410a
Ozone Depletion Potential (ODP) and Global Warming Potential (GWP)
In general, all refrigerant types are characterised by two numbers: Ozone Depletion Potential (ODP) and Global Warming Potential (GWP).
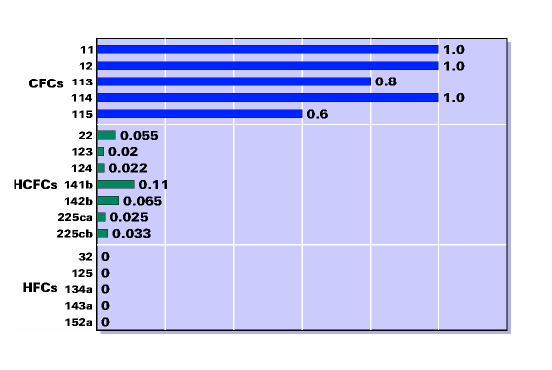
ODP values range from 0 to 1: the closest the ODP value is to 1, the more harmful the refrigerant is for the ozone layer.CFCs are generally characterised by a big ODP value, because they contain chlorine, which is accused of heavily contributing to the Ozone Depletion phenomenon. As a result, CFCs have been phased out of use nowadays.
Chart with typical refrigerants ODP values
GWP values range from 0 to several thousands: the bigger the GWP value is, the more harmful the refrigerant is for the global warming effect. In general, inorganic refrigerants like ammonia and carbon dioxide are characterised by small GWP values. In general, HCFCs have also been phased out since 2005, and only the chlorine free (zero ozone depletion) HFCs are allowed for use nowadays.
Zeotropic and azeotropic mixtures
Azeotropic mixtures are mixtures of two or more refrigerants whose vapour and liquid phases retain identical compositions over a wide range of temperatures. Typical examples of azeotropic mixtures can be seen below:
R-502 : 8.8% R22 and 51.2% R115
R-503 : 40.1% R23 and 59.9% R13
A zeotropic mixture mixture is one whose composition in liquid phase differs to that in vapour phase. The word zeotropic is a combination of Greek words zeo (meaning boiling) and tropi (meaning change)
Consequently, unlike azeotropic refrigerants, zeotropic refrigerants do not boil at constant temperatures.
Typical examples of zeotropic mixtures can be seen below:
R404a : R125/143a/134a (44%,52%,4%)
R407c : R32/125/134a (23%, 25%,
R410a : R32/125 (50%, 50%)
Many hydrocarbon gases like R170 (ethane), R290 (propane), R600 (butane), R600a (isobutane) have successfully been used as refrigerants in industrial, commercial and domestic applications.