pH definition
pH is a physical unit used to measure the degree of acidity or alkalinity of a solution. Its official definition is: the negative logarithm of hydrogen ion concentration, i.e. pH = -log[H+].
pH of a solution is measured in a scale ranging from 0 to 14. Its value is directly related to the ratio of hydrogen ion concentration in the solution (H+) to hydroxyl ion (OH-) concentration.
As pH value approaches 0, the solution becomes more acidic, i.e. the H+ concentration is greater than the OH- concentration. As pH value approaches 14, the solution becomes more alkaline (basic), i.e. the OH- concentration is greater than the H+ concentration. If equal amounts of H+ and OH- ions are present, then the solution is neutral and is characterised by a pH value of 7.
How pH is measured
A rough indication of pH can be obtained using suitable pH papers which change color with varying pH levels. However, these papers have limitations on their accuracy and as such, their use is limited.
More accurate measurements can be taken with a pH meter. A pH meter typically consists of the following elements: a pH measuring electrode (basically this is a hydrogen ion-sensitive glass bulb whose output varies with changes in the hydrogen ion concentration inside and outside the bulb), a reference electrode (its output does not vary with changes in hydrogen ion concentration) and a sample of the measured solution.
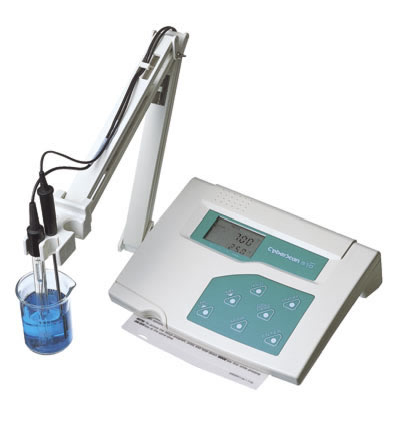
ph meter characteristics
Since pH measurement is temperature sensitive, temperature compensation is an internal element all pH meters. Temperature compensation can be either manual or automatic. In both cases, a separate temperature measurement is required.
As with all instruments, pH meters also need to be calibrated from time to time. Buffer solutions (or buffers) are typically used for calibration of pH meters. Buffer solutions are solutions that have constant pH values and are able to resist changes in pH level. Buffers are available with a wide range of pH values. Buffers can be supplied either in pre-mixed liquid form or even in dry powder capsules. pH meters typically require calibration at several specific pH values. It is highly recommended to select a buffer as close as possible to the actual pH value of the sample to be measured.