Air Compressibility Factors - Table of compressibility factor (Z factor) for air calculated at different temperature and pressure values.
Gas Compression: theory and equations - Gas compression is accompanied by change of state, which means change temperature and volume of a quantum of gas going under compression.
If the pressure of gas is raised from P1 to P2, the compression ratio for this process is defined as, P1 / P2. Theory and equations representing this change of state of gas under adiabatic compression, isothermal compression and polytropic compression of gases are discussed.
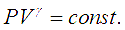
Joule Thomson Effect Definition - Understanding the Joule Thomson effect, theory behind it and how it is practically used in the vapor compression refrigeration cycle - seen in Air Conditioners, Heat Pumps etc.
Standard Temperature and Pressure Conditions - Standard temperature and pressure conditions as well as normal temperature and pressure conditions are used as reference points in thermodynamics of gases. Normally standard and normal temperature pressure conditions are used for specifying vapor volume. Universally recognized standard conditions can be easily used to specify gas volume measured at those conditions, which can be readily converted to number of moles or gas mass.
Universal Gas Constant (R) - The universal gas constant is also known by alternative names such as Ideal gas constant, molar gas constant or simply, gas constant. This constant is common for all the gases and the numerical value of this constant depends on the units used to describe pressure, temperature, and volume. Depending different combinations of these units, different value of universal gas constant are given.
Work done in a thermodynamic process - The paths followed by a system while undergoing a change of state, are termed as thermodynamic processes. Some peculiar classes of thermodynamic processes are characterized by holding some of the thermodynamic variables constant through the path of the process. Work done during such processes can be easily calculated using equations derived specifically for each of such processes including - Isobaric (constant pressure), Isochoric (constant volume), Isothermal (constant temperature), Adiabatic (no heat exchange), Isentropic (constant entropy), Isenthalpic (constant enthalpy) processes.
Add Comment