Heat Pump Definition
A heat pump is a device that transfers heat from one location to another using a refrigerant and a compressor. It can be used for both heating and cooling purposes and operates by compressing a refrigerant gas, circulating it through a heat exchanger to transfer heat, and then allowing the refrigerant to expand, which cools it down.
Check this post for detailed illustration of heat pumps.
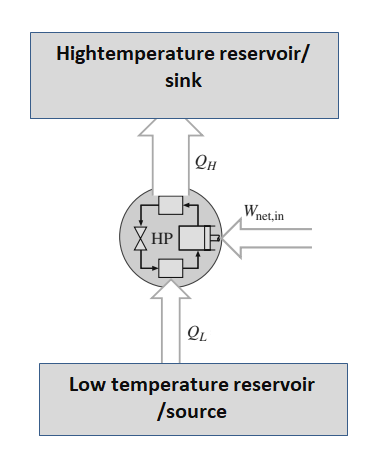
Heat Pump Cycle
The heat pump cycle for heating works by compressing and expanding a refrigerant, which allows for the transfer of heat from a low-temperature stream to a high-temperature stream. The compressor raises the temperature and pressure of the refrigerant, while the condenser releases heat to the surroundings, and the evaporator absorbs heat from the low-temperature stream.
Coefficient of performance of heat pump
The coefficient of performance of a heat pump is an important metric to consider when evaluating the performance of heat pumps. The Coefficient of Performance (COP) is a measure of the efficiency of a heat pump, and it is defined as the ratio of the amount of heat that is transferred to the high-temperature reservoir (the heat output) to the amount of work that is required to transfer the heat (the work input).
Check this post to understand coefficient of performance of a heat pump.
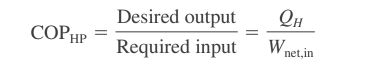
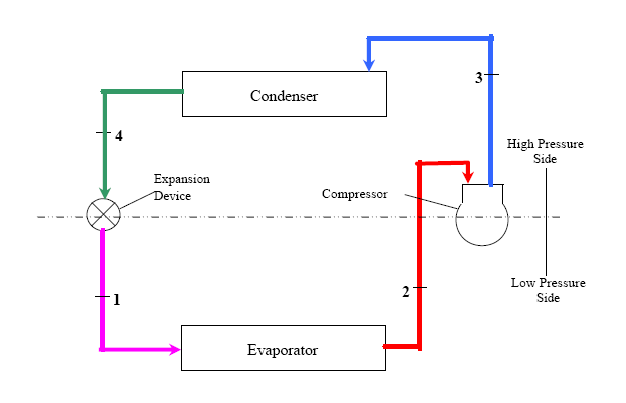
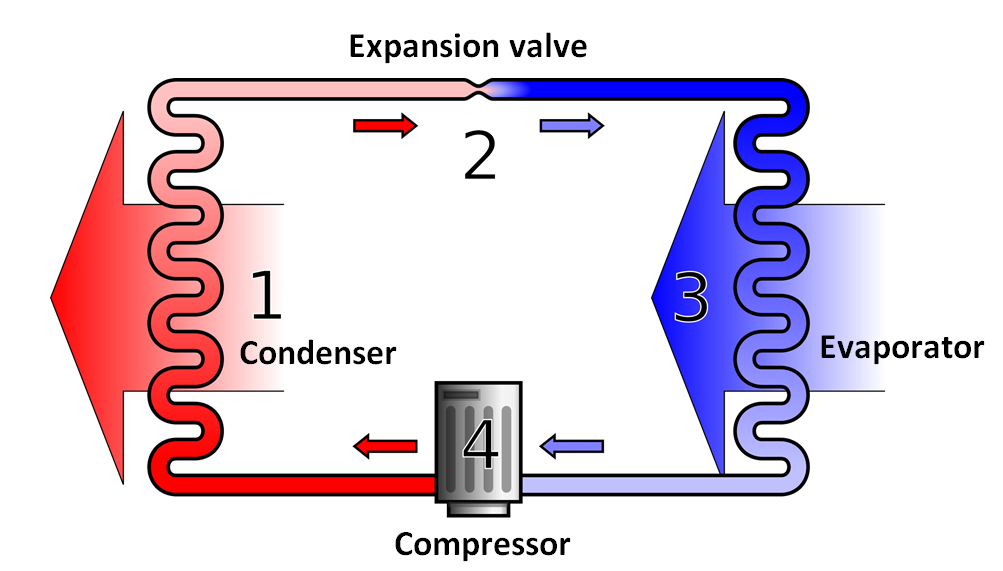
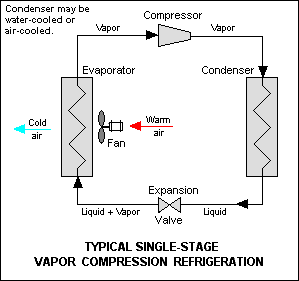
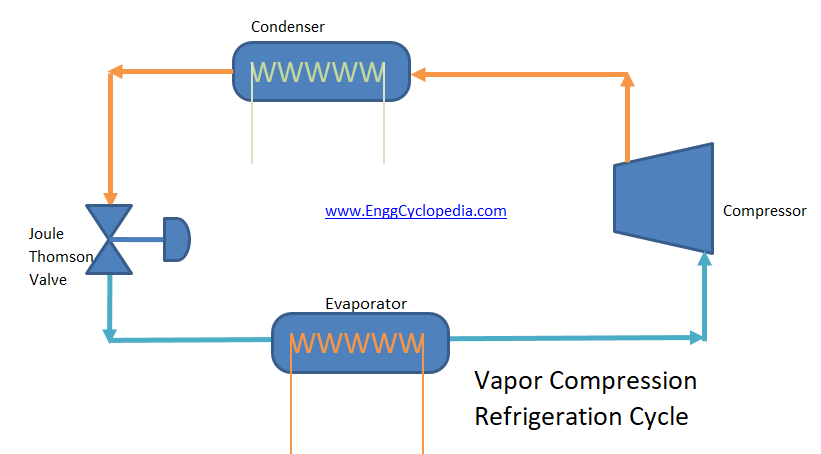
Typical Vapor Compression Refrigeration (VCR) cycle
Vapor Compression Refrigeration uses mechanical energy by repeating compression and expansion of coolant fluid to achieve cooling by Joule–Thomson effect. A majority of big refrigeration systems in use nowadays use the Vapor Compression Refrigeration (VCR) cycle.
Vapor compression refrigeration cycle is widely used in the air conditioners, heat pumps and HVAC systems.
Heat pump vs Refrigeration cycle
Both the refrigeration cycle and heat pump operate similarly by pumping heat against its natural direction. However, they have different applications. The refrigeration cycle is used to create a cooling effect in a confined space, while the heat pump is used to transfer heat from one location to another for heating or cooling purposes.
Check more detailed differentiation of both cycles.
Joule Thomson Effect
When a real gas is subjected to adiabatic expansion process, where no heat is exchanged with the surroundings, it looses its temperature. This cooling effect of gas expansion is known as the 'Joule Thomsone effect'.
Throttling or rapid expansion of a real gas across a valve tends to cool it down. This Joule Thomson effect is exploited in vapor compression refrigeration cycle.